Caco3 Solubility in Water
While the remaining amount is a mixture of protein molecules osteopontin. When you put something solid into water there are exactly two possible outcomes.
Chemistry Lesson Calcium Carbonate Solubility Lucky Sci
When set the water calculator will precipitate excess CaCO3 based on its solubility.
. The proper solubility definition is the ability to dissolve. Ie they are less soluble at higher temperature and thus tend to form deposits on hot exchanger tubes. This accounts for 95 of the entire shell structure.
Calculate the solubility of O2 in water at a partial pressure of O2 of 120 torr at 25C. PH can also affect the solubility and toxicity of chemicals and heavy metals in the water ¹². This is needed for slaked lime treatment to reduce alkalinity.
Calcium carbonate CaCO3 or CCaO3 CID 10112 - structure chemical names physical and chemical properties classification patents literature biological. If the pH of water is too high or too low the aquatic organisms living within it will die. The solubility of many compounds depends strongly on the pH of the solution.
Chromates are frequently insoluble. Soluble things dissolve and insoluble things dont dissolve. Chemistry Calculate the molar solubility of CaCO3 with Ks 510-9 in water and in a solution of NaCO3 Chemistry 01 M of pyridine to mL Chemistry.
Many salts also are less soluble at higher pH. As cooling tower water is concentrated and pH increases the tendency to pre-cipitate scale-forming salts increases. 14 If a base is soluble in water it is called an alkali.
While we can calculate the solubility by measuring each substance and following an equation. Thermodynamic properties of substances The solubility of the substances Periodic table of elements Picture of reaction. Although the solubility of silica in water is low and the dissolution rate of silicate minerals is very slow.
Alkalis Sodium hydroxide is an alkali because it dissolves in water to produce hydroxide ions. For example the anion in many sparingly soluble salts is the conjugate base of a weak acid that may become protonated in solution. Calcium carbonate is essentially insoluble in sea surface waters today.
Chemistry Using Pourbaix diagrams to calculate corrosion in water. In addition the solubility of simple binary compounds such as oxides and sulfides both strong bases is often dependent on pH. This method is impractical for residential use.
When checked the calculator takes the water after salt additions and removes CO 2 from the water until the CO 2 pressure matches atmospheric CO 2 partial pressure. The solubility rules are only for ionic solids ability to dissolve in water. CaCO3 2 HNO3 CaNO32 CO2 H2O.
NaOHaq Na aq OH aq An alkali is a soluble base which produces hydroxide ions OH aq in. Calcium carbonate has a very low solubility in pure water 15 mgL at 25C but in rainwater saturated with carbon dioxide. It either dissolves or it doesnt.
What is the molar solubility of CaCO3 at 50C in a solution pre- pared by dissolving 1000 L of CO2. Examples include PbCrO4 and BaCrO4. The solid phases of aqion are listed here in two tables.
Solution for The antifreeze in most automobile radiators is a mixture of equal volumes of ethylene glycol and water. Mechanisms of scale formation and carbon dioxide partial pressure influence Water Res 36 2002 755-763. By the time the CCD is reached all calcium carbonate.
Given that The solubility product Ksp of magnesium hydroxide is 15 10-11 We have to calculate Q. Group II carbonates CaCO3 SrCO3 and BaCO 3 are insoluble as are FeCO3 and PbCO3. Some salts have inverse temperature solubility.
The molar solubility of magnesium hydroxide in a water solution is 372x10-5 М. Chemistry Calculate the molar solubility of CaCO3 with Ks 510-9 in water and in a solution of NaCO3 Chemistry 01 M of pyridine to mL Chemistry. Silica binds to the MgOH and settles out.
1 mgL alkalinity as CaCO3 05995 mgL alkalinity as CO3 2-1 mgL alkalinity as CaCO3 12192 mgL alkalinity as HCO3 Why is pH Important. Solubility Product Constants K sp at 25C. The solubility product of CaS04 is 10-4 5.
Shells of dead calcareous plankton sinking to deeper waters are practically unaltered until reaching the lysocline the point about 35 km deep past which the solubility increases dramatically with depth and pressure. Chemical precipitation process using CaOH and Soda Ash to raise the pH to extreme levels causing precipitation of CaCO3 and MgOH. Solubility means dissolvability except that dissolvability isnt a proper science word.
Chemistry Using Pourbaix diagrams to calculate corrosion in water. When not set slaked.

Is Caco3 Soluble In Water Techiescientist

Solubility Of Calcium Carbonate Lime Scale In Water As A Function Of Ph Download Scientific Diagram

Solubility Of Calcium Carbonate Lime Scale In Water As A Function Of Ph Download Scientific Diagram

Solubility Curve Of Different Calcium Carbonate Forms Warsinger Et Download Scientific Diagram

Is Caco3 Soluble Or Insoluble In Water Youtube
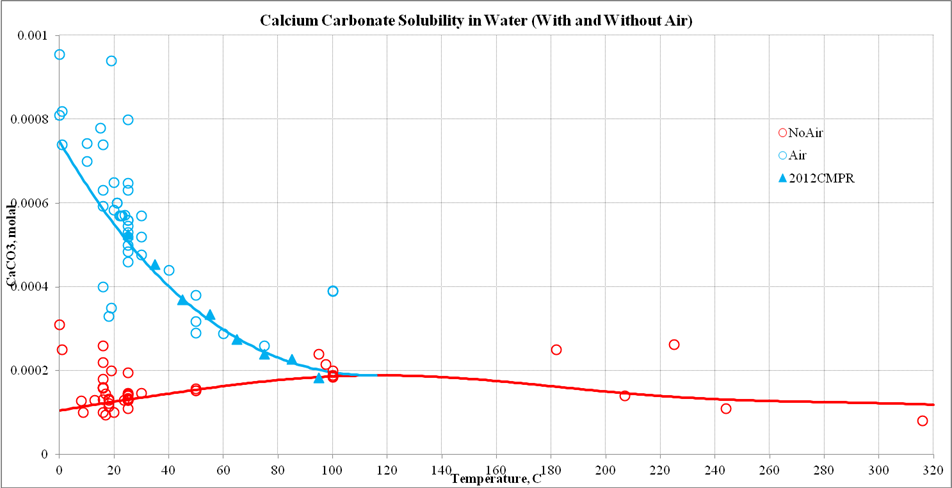
Calcium Carbonate Solubility Wiki Olisystems Com
0 Response to "Caco3 Solubility in Water"
Post a Comment